Biacore® instruments can also be used to assay the ability of small molecules to inhibit binding of macromolecules to each other. As binding of a small molecule to either the ligand or the analyte will yield a much smaller signal, measured in RUs, than the analyte itself, the overall signal should diminish as a function of the concentration of the inhibitor, if all other conditions are constant.
In the example below, we used Biacore® instrumentation to measure the association of the analyte, an MBP-fusion protein of the cytoplasmic domain of a small integral membrane protein, to the ligand, a GST-fusion protein containing a region of a large cytoskeletal protein. Binding normally occurs with a KD of ~100 nM. The small molecule inhibitor consists of a 30-mer peptide that contains the sequence on the ligand involved in binding. 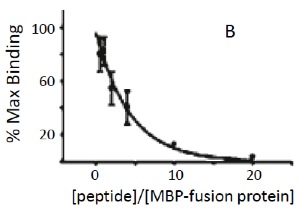
The results show that the peptide inhibits binding of the two fusion proteins to each other. Inhibition is half-maximal when the peptide is present at ~3-fold higher concentration than the analyte.
Based on these studies and parallel studies with mutants of the two proteins (numbered in purple and blue), the following molecular model of the complex form by the membrane protein with its ligand was developed.

