Before any studies can be performed on a Biacore® instrument, the chip that provides the surfaces on which binding will take place must first be prepared by attaching the ligand (e.g., an antibody, a protein, DNA, a lipid monolayer). This is called “ligand capture.” The mode of ligand capture will depend on the nature of the ligand and the molecule to which you wish to measure its binding, called the “analyte.”
If the ligand is able to bind to one of the prepared surfaces non-covalently, for example, if it is His-tagged and you are using the Ni-coated (NTA) chip or if it is biotinylated and you are using the Streptavidin (SA) chip, ligand capture simply requires that you flow a solution of your ligand over the appropriate surface until the signal, measured by the instrument in response units (RU), increases to the desired value.
Alternatively, the ligand can be captured covalently to a surface linked to carboxymethylated (CM) dextran molecules on CM4 or CM5 chips, with carbodiimide-based chemistry provided in a kit from GE Healthcare. The process, called “amine coupling,” can be direct, i.e., the ligand can be covalently coupled to the CM4 or CM5 surface, or it can be indirect. The latter is often preferable and is our method of choice for all our studies of antibody-antigen interactions. We also prefer it for studies of protein-protein interactions when we can prepare our proteins as GST or MBP chimeras in bacteria.
 In direct ligand capture, we use the coupling kit from GE to activate the CM (usually CM5) surface, and then flow a solution of the ligand over the surface until the machine registers RU in the desired range. The ligand solution is then replaced with a wash buffer, followed by a solution of ethanolamine, which inactivates any remaining sites on the surface that could react covalently with ligand or analyte. The surface is now ready for “Yes-No” or other binding studies with added analyte. Once binding studies are completed and the chip is regenerated, the surface will retain the captured ligand, because it is covalently coupled. If the ligand remains active, the chip can be reused several or even many times. As only the analyte need be added to the chip surface in repeat experiments, less ligand is required.
In direct ligand capture, we use the coupling kit from GE to activate the CM (usually CM5) surface, and then flow a solution of the ligand over the surface until the machine registers RU in the desired range. The ligand solution is then replaced with a wash buffer, followed by a solution of ethanolamine, which inactivates any remaining sites on the surface that could react covalently with ligand or analyte. The surface is now ready for “Yes-No” or other binding studies with added analyte. Once binding studies are completed and the chip is regenerated, the surface will retain the captured ligand, because it is covalently coupled. If the ligand remains active, the chip can be reused several or even many times. As only the analyte need be added to the chip surface in repeat experiments, less ligand is required.
In indirect ligand capture, we activate the CM5 chip, as above, but instead of capturing the ligand covalently, we capture an antibody. For example, to prepare for studies of binding of a mouse antibody to its antigen, we covalently link an antibody to mouse IgG to the chip surface. Before starting the experiment, we then apply a solution of the mouse antibody of interest – the ligand – to the anti-mouse chip, followed by a solution of the antigen – the analyte. As another example, to prepare for studies of binding of a GST fusion protein to another molecule, we covalently link an anti-GST antibody to the surface. We then apply a solution of the GST-fusion protein (ligand) to the anti-GST chip, followed by a solution of the (analyte) molecule of interest.
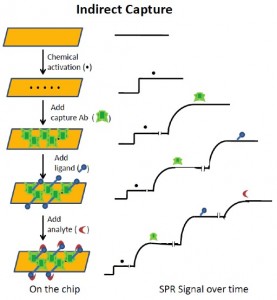 Upon regenerating a chip used for indirect capture experiments, both the analyte and ligand are washed away, leaving only the original antibody (anti-mouse, anti-GST) covalently bound to the chip surface. Unlike many ligands, these antibodies are highly stable and thus the chip can be reused dozens or even hundreds of times. In each run, however, both the captured antibody, as ligand, and its analyte must be reintroduced. This increases the time and the amount of ligand needed for the study.
Upon regenerating a chip used for indirect capture experiments, both the analyte and ligand are washed away, leaving only the original antibody (anti-mouse, anti-GST) covalently bound to the chip surface. Unlike many ligands, these antibodies are highly stable and thus the chip can be reused dozens or even hundreds of times. In each run, however, both the captured antibody, as ligand, and its analyte must be reintroduced. This increases the time and the amount of ligand needed for the study.
